At the smelter, the alumina – now a powder – goes through the Hall–Héroult electrochemical process to convert it into aluminium metal.
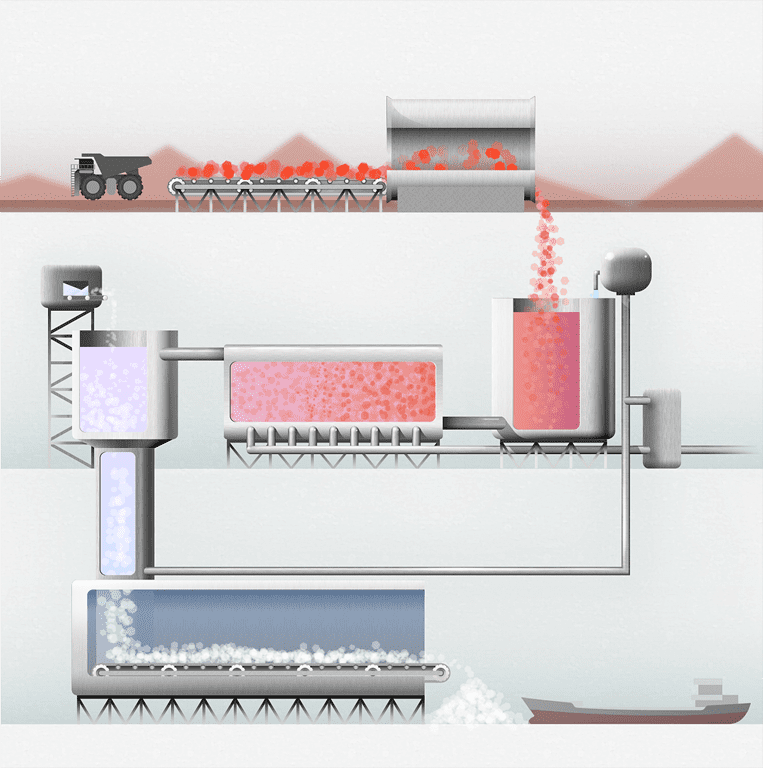
Let’s take a look at by far the most common method of obtaining alumina from bauxite: the Bayer Process. A series of controlled chemical reactions, this is how the process works:
The bauxite is washed and crushed, reducing the particle size and increasing the surface area for the upcoming digestion stage in the alumina refinery. Lime and spent liquor are added at the mills to make a pumpable slurry. Because silica can cause problems with the quality of the final product, bauxites with high levels of silica go through a process to remove as much of this impurity as possible.
Next, a hot caustic soda (sodium hydroxide) solution dissolves the aluminium-bearing minerals in the bauxite to form a sodium aluminate supersaturated solution, known as pregnant liquor. Temperatures within the digester range from 140°C to 280°C, depending on the type and composition of the alumina present in the bauxite ore.
Clarification separates the bauxite residue from the pregnant liquor via sedimentation. The bauxite residue sinks to the bottom of the settling tanks and is transferred to the washing tanks to recover the caustic soda. Further separation of the pregnant liquor from the bauxite residue is performed, utilising a series of security filters to ensure the final product is not contaminated with impurities present in the residue.
In this stage, the alumina is recovered by crystallisation from the pregnant liquor, which is supersaturated in sodium aluminate. The crystallisation process is driven by the progressive cooling of the pregnant liquor, forming small crystals of aluminium trihydroxide [Al(OH)³], which then grow and agglomerate to form larger crystals.
Finally, the precipitate mixture (known as hydrate) is fed into calciners, where it is roasted at temperatures of up to 1100°C to drive off chemically bound moisture. The alumina (Al2O³), now a white powder similar in size to sand grains, is ready for transportation to aluminium smelters or for further processing in the chemical industry. The chemical industry can use alumina in applications such as industrial and medical ceramics, sandpapers, pigments, cosmetics and pharmaceuticals.
It is important to note that cyclical steps within the Bayer Process make it more energy efficient. For example, in the precipitation stage, the spent liquor is heated and then cooled, and the resulting condensate is re-used as boiler feed water or for washing bauxite residue. Meanwhile, much of the remaining caustic soda is washed and recycled back into the digestion process.
.
The Bayer Process was developed and patented in 1887 by Austrian scientist Karl Josef Bayer.
Two to three tonnes of bauxite are required to produce one tonne of alumina, while more than 90% of the global alumina supply is used in aluminium production.
Alumina refineries tend to be located close to bauxite mines and/or ports for efficient transport of bulk products