The Hall-Héroult process, developed in 1886 independently by American Charles Martin Hall and Frenchman Paul Héroult, is the sole industrial method for the smelting of primary aluminium.
Aluminium is produced by electrolytic reduction, which happens in cells – also known as pots – in the smelter.
Smelters can contain hundreds of pots, and each pot can produce around a tonne of aluminium daily. The smelting of all primary aluminium follows the Hall-Héroult process. The process shares two names because it was developed independently in 1886 by American chemist Charles Martin Hall and French scientist Paul Héroult.
Nearly 150 years later, Hall-Héroult is still used to create liquid aluminium. How? The simple answer is that it is produced by passing an electric current through a molten mixture of cryolite, alumina, and aluminium fluoride, which turns it into pure aluminium.
The longer answer requires a little more science:
A significant direct current is fed into the line of connected pots. Each pot is a large carbon-lined metal container, forming the negative electrode (cathode). The pot contains an electrolytic bath of molten cryolite, maintained at around 960-980°C, in which alumina powder is dissolved. Aluminium fluoride is added to the solution to lower the electrolyte’s freezing point.
Large carbon blocks – from the anode production step of the process – are suspended in the solution and serve as the positive electrode (anode). The electrical current passes from the carbon anodes via the bath to the carbon cathode lining. The current then passes to the anode of the next pot, and so on through the series of pots.
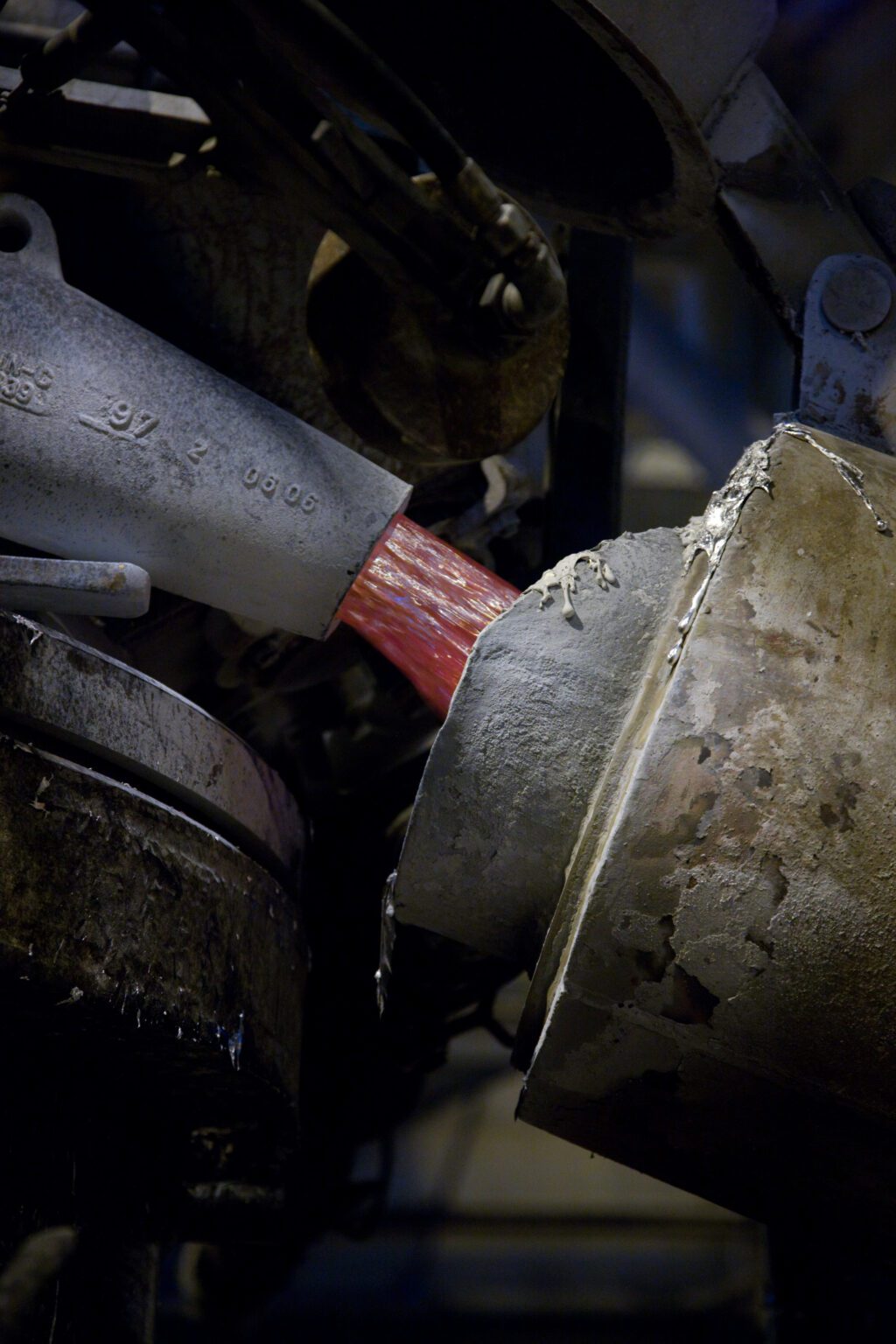
As the electrical current passes through the solution, the dissolved alumina is split into molten aluminium and oxygen. The oxygen consumes the carbon in the anode blocks to form carbon dioxide. The molten metallic aluminium sinks to the bottom of the pot, while the gaseous by-products form at the top.
The aluminium is syphoned from the pot and transported to furnaces for the next stage of its journey: dedicated casting , where it is alloyed and cast into ingots, billets, and other products.
To reduce emissions and lessen the environmental impact, fume treatment plants remove the gaseous by-products and capture hydrogen fluoride to recycle as aluminium fluoride for use in the smelting process.

Potroom Operator
Norsk Hydro (Årdal)
“Electrolysis is where it all comes together – technology, people, energy and alumina, to produce a metal that will be used and recycled and used again, almost infinitely.”